
Our Pipeline
RESEARCH & DEVELOPMENT
Servier’s aim is to make a difference in the lives of patients living with difficult and hard-to-treat diseases.
As a privately held company, Servier has the ability to think and invest for the long-term. Since entering the oncology space in 2018, Servier has rapidly and successfully built a powerful portfolio of precision therapeutics, and launched several new indications for IDH mutant cancers, including AML, MDS, glioma and CCA. Building on that success, Servier is expanding into neurology with the same dedication, scientific expertise and rigor that have defined its work in oncology.

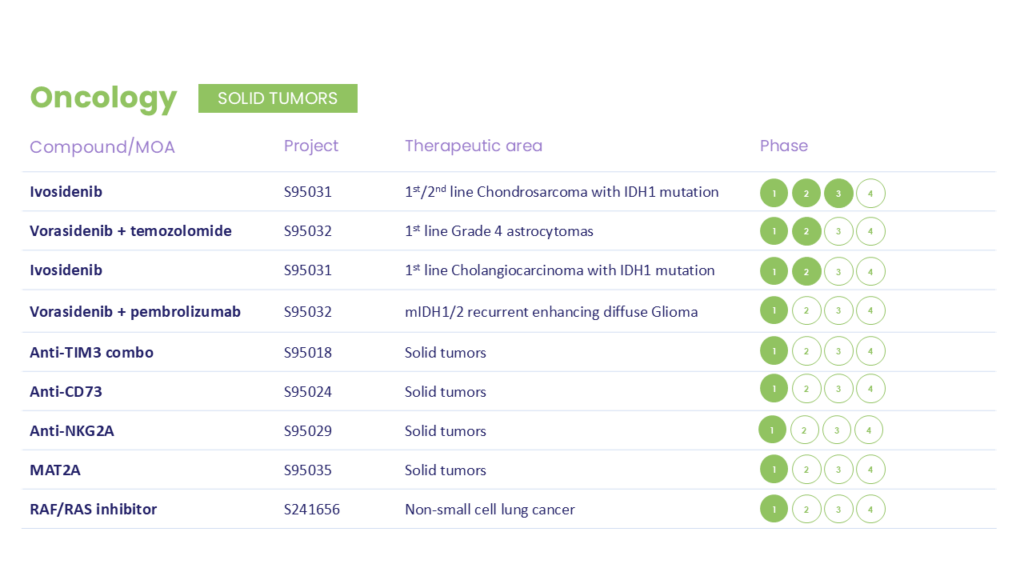
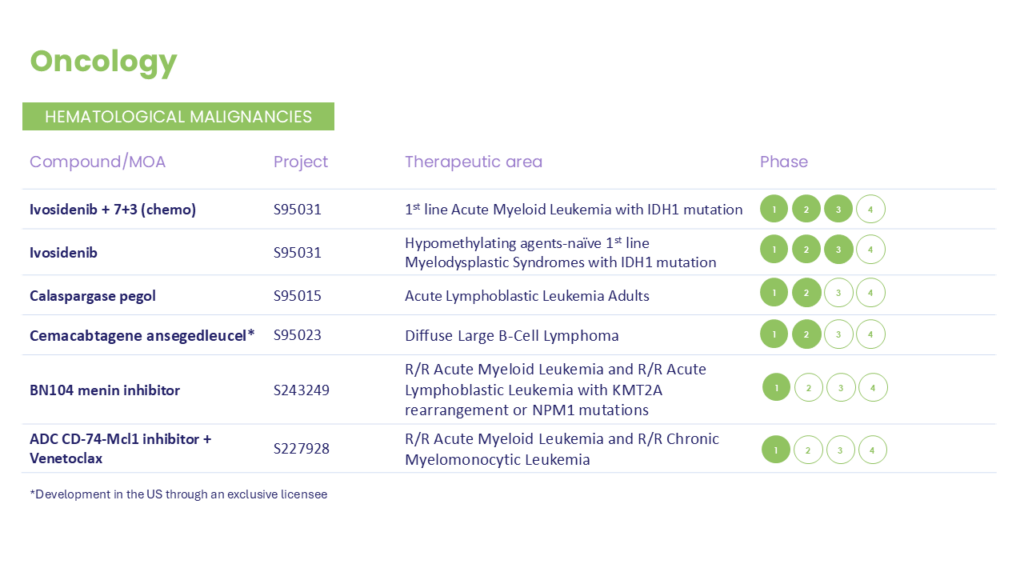

These programs are investigating treatments or outcomes that have not received approval from a health authority. The information presented is not intended to convey conclusions of safety or efficacy. There is no guarantee that the outcome of these studies will result in approval by a health authority.